After Division the New Cells Go Through the Same Thing Again What Is This Called

Mitosis in an brute cell (phases ordered counter-clockwise).



Onion (Allium) cells in different phases of the cell wheel enlarged 800 diameters.
a. not-dividing cells
b. nuclei preparing for division (spireme-stage)
c. dividing cells showing mitotic figures
e. pair of daughter-cells soon after sectionalization
In prison cell biology, mitosis () is a function of the prison cell bike in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is maintained.[1] Therefore, mitosis is also known as equational division.[2] [3] In general, mitosis is preceded past Due south stage of interphase (during which Deoxyribonucleic acid replication occurs) and is often followed past telophase and cytokinesis; which divides the cytoplasm, organelles and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components.[4] The different stages of mitosis birthday define the mitotic (Thou) phase of an fauna jail cell bike—the segmentation of the mother cell into ii daughter cells genetically identical to each other.[v]
The process of mitosis is divided into stages corresponding to the completion of one set of activities and the start of the next. These stages are preprophase (specific to plant cells), prophase, prometaphase, metaphase, anaphase, and telophase. During mitosis, the chromosomes, which have already duplicated, condense and attach to spindle fibers that pull one copy of each chromosome to contrary sides of the jail cell.[6] The result is two genetically identical daughter nuclei. The rest of the cell may then go along to divide past cytokinesis to produce ii daughter cells.[7] The different phases of mitosis tin be visualized in real fourth dimension, using live cell imaging.[8] Producing iii or more than daughter cells instead of the normal ii is a mitotic error chosen tripolar mitosis or multipolar mitosis (straight cell triplication / multiplication).[9] Other errors during mitosis tin can induce apoptosis (programmed cell decease) or cause mutations. Sure types of cancer can arise from such mutations.[ten]
Mitosis occurs just in eukaryotic cells. Prokaryotic cells, which lack a nucleus, divide by a unlike process called binary fission[ citation needed ]. Mitosis varies between organisms.[11] For example, fauna cells undergo an "open up" mitosis, where the nuclear envelope breaks down before the chromosomes separate, whereas fungi undergo a "closed" mitosis, where chromosomes divide within an intact cell nucleus.[12] Most animal cells undergo a shape change, known as mitotic cell rounding, to prefer a about spherical morphology at the get-go of mitosis. Most homo cells are produced by mitotic cell division. Important exceptions include the gametes – sperm and egg cells – which are produced by meiosis.
Discovery [edit]
Numerous descriptions of cell partition were fabricated during 18th and 19th centuries, with various degrees of accuracy.[thirteen] In 1835, the German botanist Hugo von Mohl, described prison cell division in the green algae Cladophora glomerata, stating that multiplication of cells occurs through cell partition.[14] [xv] [16] In 1838, Matthias Jakob Schleiden affirmed that "formation of new cells in their interior was a general dominion for jail cell multiplication in plants", a view afterward rejected in favour of Mohl'southward model, due to contributions of Robert Remak and others.[17]
In animal cells, jail cell division with mitosis was discovered in frog, rabbit, and cat cornea cells in 1873 and described for the first fourth dimension by the Smoothen histologist Wacław Mayzel in 1875.[18] [19]
Bütschli, Schneider and Fol might have as well claimed the discovery of the procedure presently known as "mitosis".[13] In 1873, the High german zoologist Otto Bütschli published data from observations on nematodes. A few years afterward, he discovered and described mitosis based on those observations.[20] [21] [22]
The term "mitosis", coined by Walther Flemming in 1882,[23] is derived from the Greek discussion μίτος (mitos, "warp thread").[24] [25] There are some alternative names for the procedure,[26] due east.g., "karyokinesis" (nuclear partition), a term introduced by Schleicher in 1878,[27] [28] or "equational division", proposed by Baronial Weismann in 1887.[29] However, the term "mitosis" is also used in a broad sense past some authors to refer to karyokinesis and cytokinesis together.[xxx] Soon, "equational partitioning" is more commonly used to refer to meiosis 2, the part of meiosis nearly like mitosis.[31]
Phases [edit]
Overview [edit]
The primary issue of mitosis and cytokinesis is the transfer of a parent cell's genome into two girl cells. The genome is composed of a number of chromosomes—complexes of tightly coiled Dna that incorporate genetic data vital for proper prison cell role.[32] Because each resultant daughter cell should exist genetically identical to the parent cell, the parent cell must make a copy of each chromosome before mitosis. This occurs during the S phase of interphase.[33] Chromosome duplication results in two identical sister chromatids leap together past cohesin proteins at the centromere.
When mitosis begins, the chromosomes condense and become visible. In some eukaryotes, for case animals, the nuclear envelope, which segregates the Dna from the cytoplasm, disintegrates into small vesicles. The nucleolus, which makes ribosomes in the cell, also disappears. Microtubules project from opposite ends of the prison cell, adhere to the centromeres, and align the chromosomes centrally within the cell. The microtubules so contract to pull the sister chromatids of each chromosome apart.[34] Sister chromatids at this point are called daughter chromosomes. As the cell elongates, respective daughter chromosomes are pulled toward opposite ends of the cell and condense maximally in late anaphase. A new nuclear envelope forms effectually the separated daughter chromosomes, which decondense to form interphase nuclei.
During mitotic progression, typically after the anaphase onset, the jail cell may undergo cytokinesis. In animal cells, a jail cell membrane pinches in betwixt the two developing nuclei to produce two new cells. In constitute cells, a cell plate forms between the ii nuclei. Cytokinesis does not always occur; coenocytic (a type of multinucleate condition) cells undergo mitosis without cytokinesis.
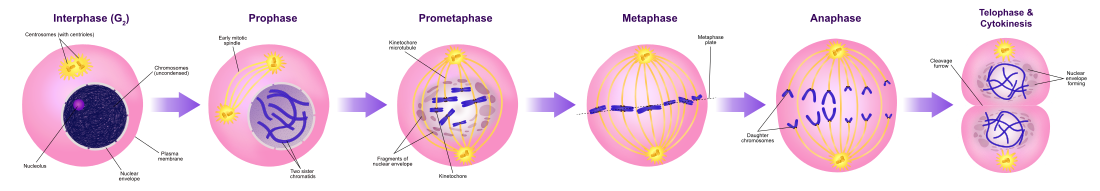
Diagram of the mitotic phases
Interphase [edit]
The mitotic phase is a relatively brusque period of the prison cell cycle. Information technology alternates with the much longer interphase, where the cell prepares itself for the process of jail cell segmentation. Interphase is divided into three phases: Grand1 (commencement gap), S (synthesis), and K2 (second gap). During all three parts of interphase, the prison cell grows by producing proteins and cytoplasmic organelles. Nevertheless, chromosomes are replicated only during the Due south phase. Thus, a cell grows (Grand1), continues to grow as it duplicates its chromosomes (S), grows more than and prepares for mitosis (One thousandii), and finally divides (M) before restarting the cycle.[33] All these phases in the jail cell bike are highly regulated past cyclins, cyclin-dependent kinases, and other prison cell bike proteins. The phases follow one some other in strict lodge and there are "checkpoints" that give the prison cell cues to proceed from ane phase to another.[35] Cells may besides temporarily or permanently leave the cell wheel and enter K0 phase to end dividing. This can occur when cells become overcrowded (density-dependent inhibition) or when they differentiate to acquit out specific functions for the organism, as is the case for man heart musculus cells and neurons. Some G0 cells have the ability to re-enter the jail cell cycle.
Dna double-strand breaks can be repaired during interphase by 2 principal processes.[36] The first process, non-homologous end joining (NHEJ), can join the two broken ends of Deoxyribonucleic acid in the G1, S and G2 phases of interphase. The 2d process, homologous recombinational repair (HRR), is more accurate than NHEJ in repairing double-strand breaks. HRR is active during the S and G2 phases of interphase when DNA replication is either partially accomplished or after it is completed, since HRR requires two adjacent homologs.
Interphase helps prepare the cell for mitotic division. It dictates whether the mitotic cell partition volition occur. It carefully stops the cell from proceeding whenever the cell's Deoxyribonucleic acid is damaged or has not completed an of import phase. The interphase is very important as it volition decide if mitosis completes successfully. It volition reduce the amount of damaged cells produced and the production of cancerous cells. A miscalculation by the key Interphase proteins could be crucial equally the latter could potentially create cancerous cells.[37] Today, more than research is being done to understand specifically how the phases stated above occur.
Mitosis [edit]

Stages of early mitosis in a vertebrate cell with micrographs of chromatids
Preprophase (plant cells) [edit]
In institute cells only, prophase is preceded past a pre-prophase stage. In highly vacuolated plant cells, the nucleus has to migrate into the center of the prison cell before mitosis can begin. This is accomplished through the formation of a phragmosome, a transverse sheet of cytoplasm that bisects the prison cell along the hereafter airplane of cell segmentation. In addition to phragmosome formation, preprophase is characterized by the germination of a band of microtubules and actin filaments (called preprophase ring) underneath the plasma membrane around the equatorial airplane of the future mitotic spindle. This ring marks the position where the cell will eventually divide. The cells of higher plants (such as the flowering plants) lack centrioles; instead, microtubules form a spindle on the surface of the nucleus and are then organized into a spindle by the chromosomes themselves, after the nuclear envelope breaks down.[38] The preprophase ring disappears during nuclear envelope breakdown and spindle germination in prometaphase.[39] : 58–67
Prophase [edit]

Condensing chromosomes. Interphase nucleus (left), condensing chromosomes (middle) and condensed chromosomes (right).

During prophase, which occurs after G2 interphase, the cell prepares to separate by tightly condensing its chromosomes and initiating mitotic spindle formation. During interphase, the genetic material in the nucleus consists of loosely packed chromatin. At the onset of prophase, chromatin fibers condense into discrete chromosomes that are typically visible at high magnification through a light microscope. In this stage, chromosomes are long, thin, and thread-like. Each chromosome has two chromatids. The two chromatids are joined at the centromere.
Gene transcription ceases during prophase and does not resume until late anaphase to early on G1 phase.[40] [41] [42] The nucleolus also disappears during early prophase.[43]
Close to the nucleus of animate being cells are structures chosen centrosomes, consisting of a pair of centrioles surrounded by a loose collection of proteins. The centrosome is the coordinating eye for the prison cell'south microtubules. A cell inherits a single centrosome at jail cell partition, which is duplicated by the prison cell earlier a new round of mitosis begins, giving a pair of centrosomes. The two centrosomes polymerize tubulin to help form a microtubule spindle apparatus. Motor proteins then button the centrosomes forth these microtubules to opposite sides of the cell. Although centrosomes help organize microtubule assembly, they are not essential for the formation of the spindle apparatus, since they are absent from plants,[38] and are not absolutely required for creature cell mitosis.[44]
Prometaphase [edit]
At the beginning of prometaphase in creature cells, phosphorylation of nuclear lamins causes the nuclear envelope to disintegrate into small membrane vesicles. Every bit this happens, microtubules invade the nuclear infinite. This is chosen open mitosis, and information technology occurs in some multicellular organisms. Fungi and some protists, such as algae or trichomonads, undergo a variation called closed mitosis where the spindle forms inside the nucleus, or the microtubules penetrate the intact nuclear envelope.[45] [46]
In late prometaphase, kinetochore microtubules begin to search for and attach to chromosomal kinetochores.[47] A kinetochore is a proteinaceous microtubule-binding construction that forms on the chromosomal centromere during tardily prophase.[47] [48] A number of polar microtubules observe and interact with respective polar microtubules from the contrary centrosome to form the mitotic spindle.[49] Although the kinetochore structure and function are not fully understood, it is known that it contains some form of molecular motor.[50] When a microtubule connects with the kinetochore, the motor activates, using energy from ATP to "crawl" up the tube toward the originating centrosome. This motor action, coupled with polymerisation and depolymerisation of microtubules, provides the pulling force necessary to afterward carve up the chromosome'south two chromatids.[50]
Metaphase [edit]
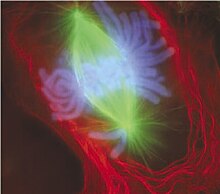
A cell in late metaphase. All chromosomes (blue) only ane accept arrived at the metaphase plate.

Afterward the microtubules have located and attached to the kinetochores in prometaphase, the two centrosomes begin pulling the chromosomes towards opposite ends of the prison cell. The resulting tension causes the chromosomes to align along the metaphase plate or equatorial airplane, an imaginary line that is centrally located betwixt the two centrosomes (at approximately the midline of the cell).[49] To ensure equitable distribution of chromosomes at the end of mitosis, the metaphase checkpoint guarantees that kinetochores are properly attached to the mitotic spindle and that the chromosomes are aligned along the metaphase plate.[51] If the prison cell successfully passes through the metaphase checkpoint, it proceeds to anaphase.
Anaphase [edit]

During anaphase A, the cohesins that bind sister chromatids together are cleaved, forming 2 identical daughter chromosomes.[52] Shortening of the kinetochore microtubules pulls the newly formed daughter chromosomes to opposite ends of the jail cell. During anaphase B, polar microtubules push button against each other, causing the cell to elongate.[53] In late anaphase, chromosomes also achieve their overall maximal condensation level, to assist chromosome segregation and the re-formation of the nucleus.[54] In most beast cells, anaphase A precedes anaphase B, but some vertebrate egg cells demonstrate the opposite gild of events.[52]
Telophase [edit]

Telophase (from the Greek word τελος pregnant "end") is a reversal of prophase and prometaphase events. At telophase, the polar microtubules proceed to lengthen, elongating the jail cell even more. If the nuclear envelope has broken downwardly, a new nuclear envelope forms using the membrane vesicles of the parent cell's one-time nuclear envelope. The new envelope forms effectually each fix of separated daughter chromosomes (though the membrane does not enclose the centrosomes) and the nucleolus reappears. Both sets of chromosomes, now surrounded by new nuclear membrane, begin to "relax" or decondense. Mitosis is complete. Each daughter nucleus has an identical set of chromosomes. Cell division may or may non occur at this time depending on the organism.
Cytokinesis [edit]

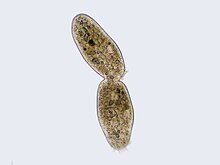
Cilliate undergoing cytokinesis, with the cleavage furrow existence clearly visible
Cytokinesis is non a phase of mitosis, but rather a separate process necessary for completing cell partition. In animal cells, a cleavage furrow (pinch) containing a contractile ring, develops where the metaphase plate used to be, pinching off the separated nuclei.[55] In both animal and institute cells, cell division is as well driven by vesicles derived from the Golgi apparatus, which movement along microtubules to the heart of the cell.[56] In plants, this structure coalesces into a jail cell plate at the center of the phragmoplast and develops into a prison cell wall, separating the ii nuclei. The phragmoplast is a microtubule structure typical for higher plants, whereas some green algae apply a phycoplast microtubule array during cytokinesis.[39] : 64–7, 328–9 Each daughter prison cell has a consummate copy of the genome of its parent cell. The cease of cytokinesis marks the end of the M-phase.
In that location are many cells where mitosis and cytokinesis occur separately, forming single cells with multiple nuclei. The about notable occurrence of this is among the fungi, slime molds, and coenocytic algae, but the phenomenon is constitute in various other organisms. Fifty-fifty in animals, cytokinesis and mitosis may occur independently, for instance during certain stages of fruit fly embryonic development.[57]
Function [edit]
Mitosis's "function" or significance relies on the maintenance of the chromosomal set; each formed cell receives chromosomes that are alike in composition and equal in number to the chromosomes of the parent cell.
Mitosis occurs in the following circumstances:
- Development and growth: The number of cells within an organism increases by mitosis. This is the basis of the development of a multicellular body from a single cell, i.e., zygote and also the basis of the growth of a multicellular torso.
- Cell replacement: In some parts of the torso, due east.g. skin and digestive tract, cells are constantly sloughed off and replaced by new ones. New cells are formed past mitosis and so are exact copies of the cells beingness replaced. In like manner, cherry blood cells have a brusque lifespan (just nigh 4 months) and new RBCs are formed by mitosis[ citation needed ].
- Regeneration: Some organisms tin regenerate torso parts. The production of new cells in such instances is achieved by mitosis. For instance, starfish regenerate lost artillery through mitosis.
- Asexual reproduction: Some organisms produce genetically like offspring through asexual reproduction. For instance, the hydra reproduces asexually past budding. The cells at the surface of hydra undergo mitosis and form a mass chosen a bud. Mitosis continues in the cells of the bud and this grows into a new private. The same sectionalization happens during asexual reproduction or vegetative propagation in plants.
Variations [edit]
Forms of mitosis [edit]
The mitosis process in the cells of eukaryotic organisms follows a similar design, but with variations in three main details. "Closed" and "open up" mitosis can be distinguished on the ground of nuclear envelope remaining intact or breaking down. An intermediate grade with partial deposition of the nuclear envelope is called "semiopen" mitosis. With respect to the symmetry of the spindle appliance during metaphase, an approximately axially symmetric (centered) shape is chosen "orthomitosis", distinguished from the eccentric spindles of "pleuromitosis", in which mitotic apparatus has bilateral symmetry. Finally, a third criterion is the location of the fundamental spindle in instance of closed pleuromitosis: "extranuclear" (spindle located in the cytoplasm) or "intranuclear" (in the nucleus).[eleven]
-
closed
intranuclear
pleuromitosis -

closed
extranuclear
pleuromitosis -
closed
orthomitosis -

semiopen
pleuromitosis -

semiopen
orthomitosis -

open
orthomitosis
Nuclear division takes place only in cells of organisms of the eukaryotic domain, every bit bacteria and archaea accept no nucleus. Leaner and archaea undergo a different blazon of partition.[ citation needed ]Within each of the eukaryotic supergroups, mitosis of the open form can be found, as well as closed mitosis, except for Excavata, which show exclusively closed mitosis.[58] Following, the occurrence of the forms of mitosis in eukaryotes:[eleven] [59]
- Closed intranuclear pleuromitosis is typical of Foraminifera, some Prasinomonadida, some Kinetoplastida, the Oxymonadida, the Haplosporidia, many fungi (chytrids, oomycetes, zygomycetes, ascomycetes), and some Radiolaria (Spumellaria and Acantharia); it seems to exist the almost primitive blazon.
- Closed extranuclear pleuromitosis occurs in Trichomonadida and Dinoflagellata.
- Closed orthomitosis is found amidst diatoms, ciliates, some Microsporidia, unicellular yeasts and some multicellular fungi.
- Semiopen pleuromitosis is typical of nigh Apicomplexa.
- Semiopen orthomitosis occurs with unlike variants in some amoebae (Lobosa) and some green flagellates (e.g., Raphidophyta or Volvox).
- Open up orthomitosis is typical in mammals and other Metazoa, and in land plants; simply it also occurs in some protists.
Errors and other variations [edit]
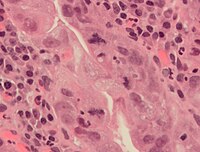
An abnormal (tripolar) mitosis (12 o'clock position) in a precancerous lesion of the stomach (H&E stain)
Errors can occur during mitosis, especially during early embryonic development in humans.[lx] During each step of mitosis, there are normally checkpoints as well that control the normal outcome of mitosis.[61] But, occasionally to almost rarely, mistakes will happen. Mitotic errors can create aneuploid cells that have besides few or too many of one or more chromosomes, a condition associated with cancer.[62] [63] Early human being embryos, cancer cells, infected or intoxicated cells tin too suffer from pathological sectionalisation into three or more than girl cells (tripolar or multipolar mitosis), resulting in severe errors in their chromosomal complements.[9]
In nondisjunction, sis chromatids neglect to separate during anaphase.[64] One girl cell receives both sister chromatids from the nondisjoining chromosome and the other cell receives none. As a result, the erstwhile cell gets three copies of the chromosome, a condition known as trisomy, and the latter will have only one copy, a condition known as monosomy. On occasion, when cells experience nondisjunction, they neglect to complete cytokinesis and retain both nuclei in one prison cell, resulting in binucleated cells.[65]
Anaphase lag occurs when the move of one chromatid is impeded during anaphase.[64] This may be caused past a failure of the mitotic spindle to properly attach to the chromosome. The lagging chromatid is excluded from both nuclei and is lost. Therefore, one of the daughter cells will be monosomic for that chromosome.
Endoreduplication (or endoreplication) occurs when chromosomes duplicate but the jail cell does not subsequently separate. This results in polyploid cells or, if the chromosomes duplicates repeatedly, polytene chromosomes.[64] [66] Endoreduplication is establish in many species and appears to be a normal function of development.[66] Endomitosis is a variant of endoreduplication in which cells replicate their chromosomes during Southward phase and enter, but prematurely terminate, mitosis. Instead of being divided into two new daughter nuclei, the replicated chromosomes are retained inside the original nucleus.[57] [67] The cells and then re-enter Grandane and S phase and replicate their chromosomes again.[67] This may occur multiple times, increasing the chromosome number with each round of replication and endomitosis. Platelet-producing megakaryocytes go through endomitosis during prison cell differentiation.[68] [69]
Amitosis in ciliates and in beast placental tissues results in a random distribution of parental alleles.
Karyokinesis without cytokinesis originates multinucleated cells called coenocytes.
Diagnostic marker [edit]

Mitosis appearances in breast cancer
In histopathology, the mitosis charge per unit (mitotic count or mitotic index) is an important parameter in various types of tissue samples, for diagnosis as well equally to further specify the aggressiveness of tumors. For case, there is routinely a quantification of mitotic count in chest cancer classification.[seventy] The mitoses must be counted in an area of the highest mitotic action. Visually identifying these areas, is hard in tumors with very high mitotic activity.[71] Besides, the detection of singular forms of mitosis can be used both as a diagnostic and prognostic mark.[ commendation needed ] For example, lag-type mitosis (not-attached condensed chromatin in the surface area of the mitotic figure) indicates loftier risk human papillomavirus infection-related Cervical cancer.[ citation needed ] In order to better the reproducibilty and accurateness of the mitotic count, automatic image analysis using deep learning-based algorithms have been proposed.[72] Even so, farther research is needed before those algorithms can be used to routine diagnostics.
-

Normal and atypical forms of mitosis in cancer cells. A, normal mitosis; B, chromatin span; C, multipolar mitosis; D, band mitosis; Eastward, dispersed mitosis; F, asymmetrical mitosis; G, lag-type mitosis; and H, micronuclei. H&E stain.
[edit]
Cell rounding [edit]

Cell shape changes through mitosis for a typical animal prison cell cultured on a flat surface. The prison cell undergoes mitotic cell rounding during spindle assembly then divides via cytokinesis. The actomyosin cortex is depicted in cherry-red, DNA/chromosomes purple, microtubules green, and membrane and retraction fibers in black. Rounding likewise occurs in live tissue, as described in the text.
In animate being tissue, most cells round upward to a near-spherical shape during mitosis.[73] [74] [75] In epithelia and epidermis, an efficient rounding process is correlated with proper mitotic spindle alignment and subsequent correct positioning of girl cells.[74] [75] [76] [77] Moreover, researchers take found that if rounding is heavily suppressed it may outcome in spindle defects, primarily pole splitting and failure to efficiently capture chromosomes.[78] Therefore, mitotic cell rounding is thought to play a protective part in ensuring accurate mitosis.[77] [79]
Rounding forces are driven by reorganization of F-actin and myosin (actomyosin) into a contractile homogeneous prison cell cortex that one) rigidifies the jail cell periphery[79] [lxxx] [81] and 2) facilitates generation of intracellular hydrostatic pressure (upwards to 10 fold higher than interphase).[82] [83] [84] The generation of intracellular pressure level is particularly critical under solitude, such as would be important in a tissue scenario, where outward forces must be produced to round upwards against surrounding cells and/or the extracellular matrix. Generation of pressure level is dependent on formin-mediated F-actin nucleation[84] and Rho kinase (Stone)-mediated myosin II contraction,[fourscore] [82] [84] both of which are governed upstream by signaling pathways RhoA and ECT2[80] [81] through the action of Cdk1.[84] Due to its importance in mitosis, the molecular components and dynamics of the mitotic actomyosin cortex is an expanse of active research.
Mitotic recombination [edit]
Mitotic cells irradiated with X-rays in the G1 phase of the cell cycle repair recombinogenic DNA amercement primarily past recombination betwixt homologous chromosomes.[85] Mitotic cells irradiated in the G2 stage repair such damages preferentially by sister-chromatid recombination.[85] Mutations in genes encoding enzymes employed in recombination cause cells to take increased sensitivity to being killed by a multifariousness of Dna dissentious agents.[86] [87] [88] These findings suggest that mitotic recombination is an adaptation for repairing DNA damages including those that are potentially lethal.
Evolution [edit]
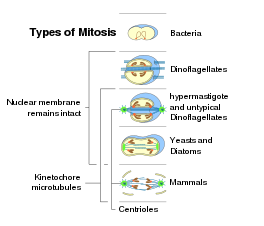
Some types of jail cell division in prokaryotes and eukaryotes
At that place are prokaryotic homologs of all the primal molecules of eukaryotic mitosis (due east.g., actins, tubulins). Being a universal eukaryotic property, mitosis probably arose at the base of the eukaryotic tree. As mitosis is less complex than meiosis, meiosis may accept arisen later mitosis.[89] Even so, sexual reproduction involving meiosis is also a primitive characteristic of eukaryotes.[90] Thus meiosis and mitosis may both have evolved, in parallel, from ancestral prokaryotic processes.
While in bacterial cell partitioning, later on duplication of DNA, two round chromosomes are attached to a special region of the prison cell membrane, eukaryotic mitosis is usually characterized by the presence of many linear chromosomes, whose kinetochores attaches to the microtubules of the spindle. In relation to the forms of mitosis, closed intranuclear pleuromitosis seems to be the most archaic type, as it is more like to bacterial division.[11]
Gallery [edit]
Mitotic cells can exist visualized microscopically by staining them with fluorescent antibodies and dyes.
-

Early prophase: Polar microtubules, shown as green strands, take established a matrix effectually the currently intact nucleus, with the condensing chromosomes in blue. The red nodules are the centromeres.
-

Early prometaphase: The nuclear membrane has merely disassembled, allowing the microtubules to quickly interact with the kinetochores, which gather on the centromeres of the condensing chromosomes.
-
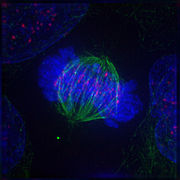
Metaphase: The centrosomes take moved to the poles of the prison cell and have established the mitotic spindle. The chromosomes have congressed at the metaphase plate.
-

Anaphase: Kinetochore microtubules pull the two sets of chromosomes autonomously, and lengthening polar microtubules push the halves of the dividing jail cell further apart, while chromosomes are condensed maximally.
-
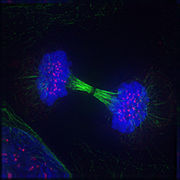
Telophase: Reversal of prophase and prometaphase events and thus completing the prison cell bicycle.
See also [edit]
- Aneuploidy
- Binary fission
- Chromosome abnormality
- Cytoskeleton
- Meiosis
- Mitogen
- Mitosis Promoting Cistron
- Mitotic bookmarking
- Motor protein
References [edit]
- ^ "Prison cell division and growth". britannica.com. ENCYCLOPÆDIA BRITANNICA. Archived from the original on 2018-x-28. Retrieved 2018-11-04 .
- ^ "4.ane: Meiosis". Biology LibreTexts. 2019-ten-01. Retrieved 2021-05-29 .
- ^ "Explain why mitosis is called equational and meiosis class 11 biology CBSE". www.vedantu.com . Retrieved 2021-05-29 .
- ^ Carter JS (2014-01-14). "Mitosis". biology.clc.uc.edu. Archived from the original on 2012-ten-27. Retrieved 2019-eleven-12 .
- ^ "Mitosis - an overview | ScienceDirect Topics". world wide web.sciencedirect.com . Retrieved 2020-eleven-24 .
- ^ "Jail cell Division: Stages of Mitosis | Larn Science at Scitable". world wide web.nature.com. Archived from the original on 2015-xi-14. Retrieved 2015-11-16 .
- ^ Maton A, Hopkins JJ, LaHart Due south, Quon Warner D, Wright Chiliad, Jill D (1997). Cells: Building Blocks of Life . New Bailiwick of jersey: Prentice Hall. pp. seventy–4. ISBN978-0-13-423476-2.
- ^ Sandoz PA (December 2019). "Image-based analysis of living mammalian cells using characterization-costless 3D refractive index maps reveals new organelle dynamics and dry out mass flux". PLOS Biology. 17 (12): e3000553. doi:x.1371/journal.pbio.3000553. PMC6922317. PMID 31856161.
- ^ a b Kalatova B, Jesenska R, Hlinka D, Dudas M (Jan 2015). "Tripolar mitosis in human cells and embryos: occurrence, pathophysiology and medical implications". Acta Histochemica. 117 (1): 111–25. doi:x.1016/j.acthis.2014.eleven.009. PMID 25554607.
- ^ Kops GJ, Weaver BA, Cleveland DW (Oct 2005). "On the road to cancer: aneuploidy and the mitotic checkpoint". Nature Reviews. Cancer. 5 (10): 773–85. doi:x.1038/nrc1714. PMID 16195750. S2CID 2515388.
- ^ a b c d Raikov IB (1994). "The diversity of forms of mitosis in protozoa: A comparative review". European Periodical of Protistology. 30 (3): 253–69. doi:x.1016/S0932-4739(11)80072-6.
- ^ De Souza CP, Osmani SA (September 2007). "Mitosis, not simply open or closed". Eukaryotic Cell. 6 (9): 1521–vii. doi:10.1128/EC.00178-07. PMC2043359. PMID 17660363.
- ^ a b Ross, Anna E. "Man Anatomy & Physiology I: A Chronology of the Description of Mitosis". Christian Brothers University. Retrieved 02 May 2018. link Archived 2016-05-12 at the Wayback Machine.
- ^ von Mohl H (1835). Ueber die Vermehrung der Pflanzenzellen durch Theilung. Inaugural-Dissertation (Thesis). Tübingen.
- ^ Karl Mägdefrau (1994), "Mohl, Hugo von", Neue Deutsche Biographie (in High german), vol. 17, Berlin: Duncker & Humblot, pp. 690–691 ; (full text online)
- ^ "Notes and memoranda: The late professor von Mohl". Quarterly Journal of Microscopical Science, v. XV, New Series, p. 178-181, 1875. link.
- ^ Weyers, Wolfgang (2002). 150 Years of cell sectionalisation. Dermatopathology: Practical & Conceptual, Vol. eight, No. 2. link Archived 2019-04-02 at the Wayback Machine
- ^ Komender J (2008). "Kilka słów o doktorze Wacławie Mayzlu i jego odkryciu" [On Waclaw Mayzel and his observation of mitotic division] (PDF). Postępy Biologii Komórki (in Smooth). 35 (3): 405–407. Archived (PDF) from the original on 2012-ten-27.
- ^ Iłowiecki M (1981). Dzieje nauki polskiej. Warszawa: Wydawnictwo Interpress. p. 187. ISBN978-83-223-1876-viii.
- ^ Bütschli, O. (1873). Beiträge zur Kenntnis der freilebenden Nematoden. Nova Acta der Kaiserlich Leopoldinisch-Carolinischen Deutschen Akademie der Naturforscher 36, one-144. link Archived 2018-08-11 at the Wayback Machine.
- ^ Bütschli, O. (1876). Studien über die ersten Entwicklungsvorgänge der Eizelle, dice Zelleilung und die Conjugation der Infusorien. Abh.d. Senckenb. Naturf. Ges. Frankfurt a. M. 10, 213-452. link Archived 2018-08-09 at the Wayback Car.
- ^ Fokin SI (2013). "Otto Bütschli (1848–1920) Where we will genuflect?" (PDF). Protistology. 8 (i): 22–35. Archived (PDF) from the original on 2014-08-08. Retrieved 2014-08-06 .
- ^ Abrupt LW (1921). Introduction To Cytology. New York: McGraw Hill Book Visitor Inc. p. 143.
- ^ "mitosis". Online Etymology Lexicon. Archived from the original on 2017-09-28. Retrieved 2019-11-12 .
- ^ μίτος . Liddell, Henry George; Scott, Robert; A Greek–English language Lexicon at the Perseus Project
- ^ Battaglia E (2009). "Caryoneme alternative to chromosome and a new caryological classification" (PDF). Caryologia. 62 (4): i–lxxx. Archived from the original (PDF) on 2016-03-04.
- ^ Schleicher West (1878). "Dice Knorpelzelltheilung". Curvation. Mirkroskop. Anat. 16: 248–300. doi:x.1007/BF02956384. S2CID 163374324. Archived from the original on 2018-08-xi.
- ^ Toepfer M. "Karyokinesis". BioConcepts. Archived from the original on 2018-05-03. Retrieved two May 2018.
- ^ Battaglia Eastward (1987). "Embryological questions: 12. Have the Polygonum and Allium types been rightly established?". Ann Bot. Rome. 45: 81–117.
p. 85: Already in 1887, Weismann gave the names Aequationstheilung to the usual cell division, and Reduktionstheilungen to the two divisions involved in the halving process of the number of Kernsegmente
- ^ Mauseth JD (1991). Botany: an Introduction to Found Biology. Philadelphia: Saunders College Publishing. ISBN9780030302220.
p. 102: Cell division is cytokinesis, and nuclear sectionalization is karyokinesis. The words "mitosis" and "meiosis" technically refer just to karyokinesis simply are often used to draw cytokinesis as well.
- ^ Cooper, Geoffrey M. (2000). "Meiosis and Fertilization". The Cell: A Molecular Approach. 2nd Edition.
- ^ Brown, Terence A. (2002). The Human Genome. Wiley-Liss.
- ^ a b Blow JJ, Tanaka TU (Nov 2005). "The chromosome bike: coordinating replication and segregation. 2d in the cycles review serial". EMBO Reports. half dozen (xi): 1028–34. doi:10.1038/sj.embor.7400557. PMC1371039. PMID 16264427.
- ^ Zhou J, Yao J, Joshi HC (September 2002). "Zipper and tension in the spindle assembly checkpoint". Journal of Cell Scientific discipline. 115 (Pt 18): 3547–55. doi:10.1242/jcs.00029. PMID 12186941.
- ^ Biology Online (28 April 2020). "Mitosis". Biology Online.
- ^ Shibata A (2017). "Regulation of repair pathway choice at two-ended DNA double-strand breaks". Mutat Res. 803–805: 51–55. doi:10.1016/j.mrfmmm.2017.07.011. PMID 28781144.
- ^ Bernat, R. L.; Borisy, 1000. Thou.; Rothfield, Northward. F.; Earnshaw, W. C. (1990-10-01). "Injection of anticentromere antibodies in interphase disrupts events required for chromosome move at mitosis". The Periodical of Cell Biology. 111 (4): 1519–1533. doi:ten.1083/jcb.111.4.1519. ISSN 0021-9525. PMC2116233. PMID 2211824.
- ^ a b Lloyd C, Chan J (February 2006). "Not and so divided: the mutual basis of plant and brute cell division". Nature Reviews. Molecular Prison cell Biology. 7 (2): 147–52. doi:10.1038/nrm1831. PMID 16493420. S2CID 7895964.
- ^ a b Raven PH, Evert RF, Eichhorn SE (2005). Biology of Plants (7th ed.). New York: West. H. Freeman and Co. ISBN978-0716710073.
- ^ Prasanth KV, Sacco-Bubulya PA, Prasanth SG, Spector DL (March 2003). "Sequential entry of components of the gene expression machinery into daughter nuclei". Molecular Biological science of the Cell. 14 (iii): 1043–57. doi:10.1091/mbc.E02-10-0669. PMC151578. PMID 12631722.
- ^ Kadauke S, Blobel GA (April 2013). "Mitotic bookmarking by transcription factors". Epigenetics & Chromatin. 6 (1): vi. doi:x.1186/1756-8935-6-six. PMC3621617. PMID 23547918.
- ^ Prescott DM, Bender MA (March 1962). "Synthesis of RNA and protein during mitosis in mammalian tissue civilization cells". Experimental Jail cell Inquiry. 26 (2): 260–8. doi:10.1016/0014-4827(62)90176-3. PMID 14488623.
- ^ Olson MO (2011). The Nucleolus. Vol. xv of Protein Reviews. Berlin: Springer Scientific discipline & Business concern Media. p. xv. ISBN9781461405146.
- ^ Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (June 2006). "Flies without centrioles". Cell. 125 (7): 1375–86. doi:ten.1016/j.cell.2006.05.025. PMID 16814722. S2CID 2080684.
- ^ Heywood P (June 1978). "Ultrastructure of mitosis in the chloromonadophycean alga Vacuolaria virescens". Periodical of Cell Science. 31: 37–51. doi:10.1242/jcs.31.1.37. PMID 670329.
- ^ Ribeiro KC, Pereira-Neves A, Benchimol K (June 2002). "The mitotic spindle and associated membranes in the closed mitosis of trichomonads". Biology of the Prison cell. 94 (3): 157–72. doi:10.1016/S0248-4900(02)01191-seven. PMID 12206655. S2CID 29081466.
- ^ a b Chan GK, Liu ST, Yen TJ (November 2005). "Kinetochore structure and office". Trends in Cell Biology. 15 (11): 589–98. doi:10.1016/j.tcb.2005.09.010. PMID 16214339.
- ^ Cheeseman IM, Desai A (Jan 2008). "Molecular compages of the kinetochore-microtubule interface". Nature Reviews. Molecular Jail cell Biological science. 9 (1): 33–46. doi:ten.1038/nrm2310. PMID 18097444. S2CID 34121605.
- ^ a b Winey G, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH, McDonald KL, McIntosh JR (June 1995). "Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle". The Periodical of Prison cell Biology. 129 (6): 1601–15. doi:10.1083/jcb.129.6.1601. PMC2291174. PMID 7790357.
- ^ a b Maiato H, DeLuca J, Salmon ED, Earnshaw WC (Nov 2004). "The dynamic kinetochore-microtubule interface" (PDF). Journal of Prison cell Science. 117 (Pt 23): 5461–77. doi:10.1242/jcs.01536. PMID 15509863. S2CID 13939431. Archived (PDF) from the original on 2017-08-eighteen. Retrieved 2018-04-20 .
- ^ Chan GK, Yen TJ (2003). "The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic get out". Progress in Cell Cycle Research. 5: 431–9. PMID 14593737.
- ^ a b FitzHarris 1000 (March 2012). "Anaphase B precedes anaphase A in the mouse egg" (PDF). Current Biology. 22 (5): 437–44. doi:x.1016/j.cub.2012.01.041. PMID 22342753. Archived (PDF) from the original on 2018-07-24. Retrieved 2019-09-17 .
- ^ Miller KR, Levine J (2000). "Anaphase". Biology (5th ed.). Pearson Prentice Hall. pp. 169–seventy. ISBN978-0-thirteen-436265-6.
- ^ European Molecular Biology Laboratory (12 June 2007). "Chromosome condensation through mitosis". Science Daily. Archived from the original on thirteen June 2007. Retrieved 4 Oct 2020.
- ^ Glotzer One thousand (March 2005). "The molecular requirements for cytokinesis". Scientific discipline. 307 (5716): 1735–ix. Bibcode:2005Sci...307.1735G. doi:10.1126/science.1096896. PMID 15774750. S2CID 34537906.
- ^ Albertson R, Riggs B, Sullivan Westward (Feb 2005). "Membrane traffic: a driving forcefulness in cytokinesis". Trends in Cell Biology. fifteen (2): 92–101. doi:10.1016/j.tcb.2004.12.008. PMID 15695096.
- ^ a b Lilly MA, Duronio RJ (April 2005). "New insights into jail cell cycle command from the Drosophila endocycle". Oncogene. 24 (17): 2765–75. doi:10.1038/sj.onc.1208610. PMID 15838513.
- ^ Boettcher B, Barral Y (2013). "The cell biology of open and closed mitosis". Nucleus. iv (3): 160–5. doi:10.4161/nucl.24676. PMC3720745. PMID 23644379.
- ^ R. Desalle, B. Schierwater: Key Transitions in Animal Evolution. CRC Press, 2010, p. 12, link Archived 2019-01-02 at the Wayback Machine.
- ^ Mantikou Due east, Wong KM, Repping Southward, Mastenbroek S (Dec 2012). "Molecular origin of mitotic aneuploidies in preimplantation embryos". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1822 (12): 1921–30. doi:10.1016/j.bbadis.2012.06.013. PMID 22771499.
- ^ Wassmann, Katja; Benezra, Robert (2001-02-01). "Mitotic checkpoints: from yeast to cancer". Current Opinion in Genetics & Development. 11 (1): 83–90. doi:10.1016/S0959-437X(00)00161-one. ISSN 0959-437X. PMID 11163156.
- ^ Draviam VM, Xie S, Sorger PK (April 2004). "Chromosome segregation and genomic stability". Current Opinion in Genetics & Evolution. 14 (2): 120–5. doi:10.1016/j.gde.2004.02.007. PMID 15196457.
- ^ Santaguida Southward, Amon A (August 2015). "Short- and long-term effects of chromosome mis-segregation and aneuploidy". Nature Reviews. Molecular Cell Biology. sixteen (8): 473–85. doi:10.1038/nrm4025. hdl:1721.1/117201. PMID 26204159. S2CID 205495880.
- ^ a b c Iourov IY, Vorsanova SG, Yurov YB (2006). "Chromosomal Variations in Mammalian Neuronal Cells: Known Facts and Attractive Hypotheses". In Jeon KJ (ed.). International Review Of Cytology: A Survey of Jail cell Biological science. Vol. 249. Waltham, MA: Academic Printing. p. 146. ISBN9780080463506.
- ^ Shi Q, King RW (October 2005). "Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human jail cell lines". Nature. 437 (7061): 1038–42. Bibcode:2005Natur.437.1038S. doi:10.1038/nature03958. PMID 16222248. S2CID 1093265.
- ^ a b Edgar BA, Orr-Weaver TL (May 2001). "Endoreplication cell cycles: more for less". Prison cell. 105 (iii): 297–306. doi:ten.1016/S0092-8674(01)00334-8. PMID 11348589. S2CID 14368177.
- ^ a b Lee HO, Davidson JM, Duronio RJ (November 2009). "Endoreplication: polyploidy with purpose". Genes & Development. 23 (21): 2461–77. doi:ten.1101/gad.1829209. PMC2779750. PMID 19884253.
- ^ Italiano JE, Shivdasani RA (June 2003). "Megakaryocytes and beyond: the nascency of platelets". Journal of Thrombosis and Haemostasis. 1 (6): 1174–82. doi:10.1046/j.1538-7836.2003.00290.x. PMID 12871316. S2CID 24325966.
- ^ Vitrat Due north, Cohen-Solal K, Pique C, Le Couedic JP, Norol F, Larsen AK, Katz A, Vainchenker W, Debili N (May 1998). "Endomitosis of homo megakaryocytes are due to abortive mitosis". Claret. 91 (ten): 3711–23. doi:x.1182/claret.V91.ten.3711. PMID 9573008.
- ^ "Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type)". Stanford University School of Medicine. Archived from the original on 2019-09-xi. Retrieved 2019-ten-02 .
- ^ Bertram CA, Aubreville M, Gurtner C, Bartel A, Corner SM, Dettwiler M, et al. (March 2020). "Computerized Calculation of Mitotic Count Distribution in Canine Cutaneous Mast Cell Tumor Sections: Mitotic Count Is Area Dependent" (PDF). Veterinary Pathology. 57 (2): 214–226. doi:ten.1177/0300985819890686. PMID 31808382. S2CID 208767801.
- ^ Bertram, Christof A; Aubreville, Marc; Donovan, Taryn A; Bartel, Alexander; Wilm, Frauke; Marzahl, Christian; Assenmacher, Charles-Antoine; Becker, Kathrin; Bennett, Mark; Corner, Sarah; Cossic, Brieuc; Denk, Daniela; Dettwiler, Martina; Gonzalez, Beatriz Garcia; Gurtner, Corinne; Haverkamp, Ann-Kathrin; Heier, Annabelle; Lehmbecker, Annika; Merz, Sophie; Noland, Erika L; Plog, Stephanie; Schmidt, Anja; Sebastian, Franziska; Sledge, Dodd G; Smedley, Rebecca C; Tecilla, Marco; Thaiwong, Tuddow; Fuchs-Baumgartinger, Andrea; Meuten, Donald J; Breininger, Katharina; Kiupel, Matti; Maier, Andreas; Klopfleisch, Robert (2021). "Figurer-assisted mitotic count using a deep learning–based algorithm improves interobserver reproducibility and accuracy". Veterinary Pathology. doi:10.1177/03009858211067478. PMID 34965805. S2CID 245567911.
- ^ Sauer FC (1935). "Mitosis in the neural tube". Periodical of Comparative Neurology. 62 (2): 377–405. doi:10.1002/cne.900620207. S2CID 84960254.
- ^ a b Meyer EJ, Ikmi A, Gibson MC (March 2011). "Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia". Current Biology. 21 (half dozen): 485–91. doi:ten.1016/j.cub.2011.02.002. PMID 21376598.
- ^ a b Luxenburg C, Pasolli HA, Williams SE, Fuchs E (March 2011). "Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation". Nature Prison cell Biology. 13 (3): 203–14. doi:10.1038/Ncb2163. PMC3278337. PMID 21336301.
- ^ Nakajima Y, Meyer EJ, Kroesen A, McKinney SA, Gibson MC (August 2013). "Epithelial junctions maintain tissue architecture by directing planar spindle orientation". Nature. 500 (7462): 359–62. Bibcode:2013Natur.500..359N. doi:10.1038/nature12335. PMID 23873041. S2CID 4418619.
- ^ a b Cadart C, Zlotek-Zlotkiewicz E, Le Berre Thousand, Piel One thousand, Matthews HK (April 2014). "Exploring the function of jail cell shape and size during mitosis". Developmental Cell. 29 (2): 159–69. doi:10.1016/j.devcel.2014.04.009. PMID 24780736.
- ^ Lancaster OM, Le Berre Grand, Dimitracopoulos A, Bonazzi D, Zlotek-Zlotkiewicz East, Picone R, Duke T, Piel M, Baum B (May 2013). "Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation". Developmental Jail cell. 25 (three): 270–83. doi:10.1016/j.devcel.2013.03.014. PMID 23623611.
- ^ a b Lancaster OM, Baum B (October 2014). "Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis". Seminars in Prison cell & Developmental Biology. 34: 109–15. doi:10.1016/j.semcdb.2014.02.015. PMID 24607328.
- ^ a b c Maddox Every bit, Burridge K (January 2003). "RhoA is required for cortical retraction and rigidity during mitotic cell rounding". The Journal of Prison cell Biology. 160 (2): 255–65. doi:ten.1083/jcb.200207130. PMC2172639. PMID 12538643.
- ^ a b Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B (August 2012). "Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression". Developmental Cell. 23 (two): 371–83. doi:ten.1016/j.devcel.2012.06.003. PMC3763371. PMID 22898780.
- ^ a b Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA (January 2011). "Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding". Nature. 469 (7329): 226–30. Bibcode:2011Natur.469..226S. doi:10.1038/nature09642. PMID 21196934. S2CID 4425308.
- ^ Fischer-Friedrich E, Hyman AA, Jülicher F, Müller DJ, Helenius J (August 2014). "Quantification of surface tension and internal pressure generated by single mitotic cells". Scientific Reports. 4 (6213): 6213. Bibcode:2014NatSR...4E6213F. doi:10.1038/srep06213. PMC4148660. PMID 25169063.
- ^ a b c d Ramanathan SP, Helenius J, Stewart MP, Cattin CJ, Hyman AA, Muller DJ (February 2015). "Cdk1-dependent mitotic enrichment of cortical myosin 2 promotes cell rounding against solitude". Nature Prison cell Biology. 17 (2): 148–59. doi:10.1038/ncb3098. PMID 25621953. S2CID 5208968.
- ^ a b Kadyk LC, Hartwell LH (October 1992). "Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae". Genetics. 132 (two): 387–402. doi:x.1093/genetics/132.2.387. PMC1205144. PMID 1427035.
- ^ Botthof JG, Bielczyk-Maczyńska E, Ferreira Fifty, Cvejic A (May 2017). "rad51 leads to Fanconi anemia-like symptoms in zebrafish". Proceedings of the National Academy of Sciences of the United states of america of America. 114 (22): E4452–E4461. doi:10.1073/pnas.1620631114. PMC5465903. PMID 28512217.
Here nosotros provide in vivo evidence that the decrease in HSPC numbers in developed fish indeed stems from a combination of decreased proliferation and increased apoptosis during embryonic development. This defect appears to be mediated via p53(10), equally our p53/rad51 double mutants did non display any observable hematological defects in embryos or adults.
- ^ Stürzbecher HW, Donzelmann B, Henning Due west, Knippschild U, Buchhop Due south (Apr 1996). "p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction". The EMBO Journal. 15 (8): 1992–2002. doi:ten.1002/j.1460-2075.1996.tb00550.ten. PMC450118. PMID 8617246.
- ^ Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, Ogawa H, et al. (January 1998). "Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell decease". The EMBO Journal. 17 (2): 598–608. doi:x.1093/emboj/17.2.598. PMC1170409. PMID 9430650.
- ^ Wilkins AS, Holliday R (January 2009). "The evolution of meiosis from mitosis". Genetics. 181 (1): iii–12. doi:10.1534/genetics.108.099762. PMC2621177. PMID 19139151.
- ^ Bernstein, H., Bernstein, C. Evolutionary origin and adaptive function of meiosis. In "Meiosis", Intech Publ (Carol Bernstein and Harris Bernstein editors), Chapter 3: 41-75 (2013).
Farther reading [edit]
- Morgan DL (2007). The prison cell cycle: principles of control. London: Published by New Science Press in clan with Oxford University Press. ISBN978-0-9539181-2-6.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Mitosis". Molecular Biology of the Cell (4th ed.). Garland Science. Retrieved 2006-01-22 .
- Campbell Northward, Reece J (Dec 2001). "The Prison cell Cycle". Biology (6th ed.). San Francisco: Benjamin Cummings/Addison-Wesley. pp. 217–224. ISBN978-0-8053-6624-2.
- Cooper Thousand (2000). "The Events of Yard Phase". The Prison cell: A Molecular Approach (second ed.). Sinaeur Associates, Inc. Retrieved 2006-01-22 .
- Freeman S (2002). "Prison cell Partitioning". Biological Science . Upper Saddle River, NJ: Prentice Hall. pp. 155–174. ISBN978-0-thirteen-081923-9.
- Lodish H, Berk A, Zipursky 50, Matsudaira P, Baltimore D, Darnell J (2000). "Overview of the Cell Cycle and Its Control". Molecular Prison cell Biology (4th ed.). West. H. Freeman. Retrieved 2006-01-22 .
External links [edit]
| | Wikimedia Eatables has media related to Mitosis. |
- A Wink blitheness comparison Mitosis and Meiosis
- Khan University, lecture
- Studying Mitosis in Cultured Mammalian Cells
- Full general K-12 classroom resources for Mitosis
- The Cell-Cycle Ontology
- WormWeb.org: Interactive Visualization of the C. elegans Cell Lineage – Visualize the entire cell lineage tree and all of the jail cell divisions of the nematode C. elegans
keblethearegaven2001.blogspot.com
Source: https://en.wikipedia.org/wiki/Mitosis
0 Response to "After Division the New Cells Go Through the Same Thing Again What Is This Called"
Post a Comment